Citation
Okoronkwo, M. U.; Balonis, M.; Katz, L.; Juenger, M.; Sant, G. J. Environ. Manage. 2018, 217, 278-287.
Okoronkwo, M. U.; Balonis, M.; Katz, L.; Juenger, M.; Sant, G. J. Environ. Manage. 2018, 217, 278-287.
|
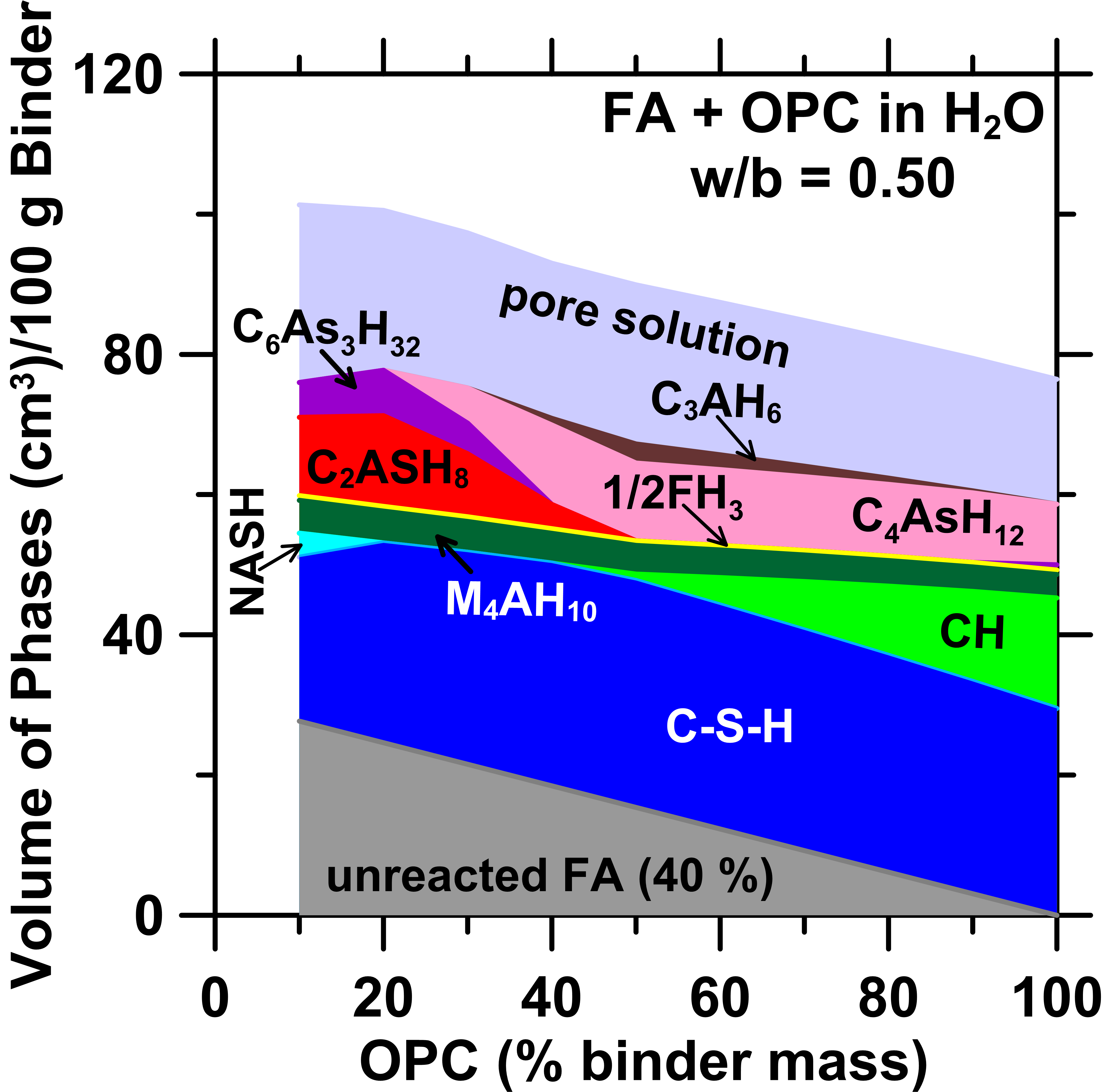
Cementitious binders are often used to immobilize industrial wastes such as residues of coal combustion. Such immobilization stabilizes wastes that contain contaminants by chemical containment, i.e., by uptake of contaminants into the cementitious reaction products. Expectedly, the release (“leachability”) of contaminants is linked to: (i) the stability of the matrix (i.e., its resistance to decomposition on exposure to water), and, (ii) its porosity, which offers a pathway for the intrusion of water and egress of contaminant species. To examine the effects of the matrix chemistry on its suitability for immobilization, an equilibrium thermodynamics-based approach is demonstrated for cementitious formulations based on: ordinary portland cement (OPC), calcium aluminate cement (CAC) and alkali activated fly ash (AFA) binding agents. First, special focus is placed on computing the equilibrium phase assemblages using the bulk reactant compositions as an input. Second, the matrix’s stability is assessed by simulating leaching that is controlled by progressive dissolution and precipitation of solids across a range of liquid (leachant)-to-(reaction product) solid (l/s) ratios and leachant pH’s; e.g., following the LEAF 1313 and 1316 protocols. The performance of each binding formulation is evaluated based on the: (i) relative ability of the reaction products to chemically bind the contaminant(s), (ii) porosity of the matrix which correlates to its hydraulic conductivity, and, (iii) the extent of matrix degradation that follows leaching and which impact the rate and extent of release of potential contaminants. In this manner, the approach enables rapid, parametric assessment of a wide-range of stabilization solutions with due consideration of the matrix’s mineralogy, porosity, and the leaching (exposure) conditions. |