Citation
Falzone, G.; Balonis, M.; Bentz, D.; Jones, S.; Sant, G. Cement and Concrete Research 2017, 101, 82-92.
Falzone, G.; Balonis, M.; Bentz, D.; Jones, S.; Sant, G. Cement and Concrete Research 2017, 101, 82-92.
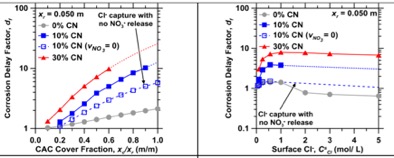
Chloride induced corrosion is a major cause of degradation of reinforced concrete infrastructure. While the binding of chloride ions (Cl-) by cementitious phases is known to delay corrosion, this approach has not been systematically exploited as a mechanism to increase structural service-life. Recently, Falzone et al. [Cement and Concrete Research 72, 54-68 (2015)] proposed calcium aluminate cement (CAC) formulations containing NO3-AFm as anion-exchange coatings capable of binding large quantities of Cl-, while simultaneously releasing corrosion-inhibiting NO3- species. To examine the viability of this concept, Cl- binding isotherms and ion-diffusion coefficients of a series of hydrated CAC formulations containing admixed Ca(NO3)2 (CN) were quantified. This data was input into a multi-species Nernst-Planck (NP) formulation and solved for a typical bridge-deck geometry using the finite element method (FEM). For exposure conditions corresponding to seawater, the results indicate that Cl- scavenging CAC coatings (i.e., top-layers) can significantly delay the time to corrosion (e.g., 5 ≤ df ≤ 10, where df is the steel corrosion initiation delay factor [unitless]) as compared to traditional OPC-based systems for a fixed cover depth; as identified by thresholds of Cl-/OH- or Cl-/NO3- (molar) ratios in solution. The roles of hindered ionic diffusion, and the passivation of the reinforcing steel rendered by NO3- are also discussed.